- 小鼠可溶性晚期糖基化终末产物受体(sRAGE)elisa试剂...
- Samwon Co.ltd.出口数据及联系方式
- RNAi转染试剂龙虎榜——为你的RNAi实验选择合适的转染试...
- 准备大组织的厚切片进行β半乳糖苷酶染色
- 液氮罐如何保存标本
- 免费待测小鼠碱性成纤维细胞生长因子(bFGF)ELISA试剂...
- www.doverchem.com # Dover Chem...
- BracebridgeCedar Lane Motel的点评...
- 全自动电泳仪咨询报告_20202025年中国全自动电泳仪行业...
- 【求助】siRNA沉默的效果是暂时的?_实验方法_
- An integrated doublefiltration...
- Chemtronics | Electronic Maint...
- [07-25]大家做5'RACE都用的什么试剂盒
- [08-02]有人不用试剂盒做出5’RACE吗?
- [07-19]关于clontech的smart race试剂盒拉5‘端的问题
- [07-22]验证miRNA对靶基因剪切位点的RLMRACE的效率问题 分子生物...
- [10-01]GeneRacer Advanced RACE Kit | Thermo Fisher Scientific US
- [04-17]各种5RACE试剂盒优缺点RACE+Introduction
- [08-01]RACE技术的原理和操作
- [10-01]TaKaRa 质粒DNA小量纯化试剂盒说明书
- [10-01]求助——哪种RACE试剂盒好,多少钱啊 生物科学论坛学术...
An integrated doublefiltration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bl
Thankyouforvisitingnature.com.YouareusingabrowserversionwithlimitedsupportforCSS.Toobtainthebestexperience,werecommendyouuseamoreuptodatebrowser(orturnoffcompatibilitymodeinInternetExplorer).Inthemeantime,toensurecontinuedsupport,wearedisplayingthesitewithoutstylesandJavaScript.
Abstract
Extracellularvesicles(EVs),includingexosomesandmicrovesicles,arepresentinavarietyofbodilyfluids,andtheconcentrationofthesesub-cellularvesiclesandtheirassociatedbioMarkers(proteins,nucleicacids,andlipids)canbeusedtoaidclinicaldiagnosis.AlthoughultracentrifugationiscommonlyusedforisolationofEVs,itishighlytime-consuming,labor-intensiveandinstrument-dependentforbothresearchlaboratoriesandclinicalsettings.Here,wedevelopedanintegrateddouble-filtrationmicrofluidicdevicethatisolatedandenrichedEVswithasizerangeof30鈥?00鈥塶mfromurine,andsubsequentlyquantifiedtheEVsviaamicrochipELISA.OurresultsshowedthattheconcentrationofurinaryEVswassignificantlyelevatedinbladdercancerpatients(n鈥?鈥?6)comparedtohealthycontrols(n鈥?鈥?).Receiveroperatingcharacteristic(ROC)analysisdemonstratedthatthisintegratedEVdouble-filtrationdevicehadasensitivityof81.3%ataspecificityof90%(16bladdercancerpatientsand8healthycontrols).Thus,thisintegrateddevicehasgreatpotentialtobeusedinconjunctionwithurinecytologyandcystoscopytoimproveclinicaldiagnosisofbladdercancerinclinicsandatpoint-of-care(POC)settings.
Introduction
Bladdercancerranksthesecondmostcommonmalignancyoccurringtothegenitourinarysystem1,2.Theincidenceofbladdercanceris20.1per100,000intheUS3,whereasitis27per100,000menand6per100,000womeninEuropeanUnion4.Recentstatisticaldatarevealthattheincidenceofbladdercanceris80.5per100,000inChina5.Pathologically,bladdercancerisclassifiedintotwogroups:superficialtumors(70%)andmuscle-invasive(30%)tumors,whichoftenrecurafterintravesicaltherapyorrequireradicalcystoprostatectomy6.Ontheotherhand,the5-yearsurvivalrateofbladdercanceriscloselycorrelatedwithclinicalstaging.Forinsituandlocalizedbladdercancer,the5-yearsurvivalraterangesfrom70.2鈥?5.9%,anditdropsto5.2鈥?4.5%whenbladdercancerbecomesregionalanddistant7.Sinceapproximately80鈥?0%ofbladdercancerpatientsexperienceonlywithgrosspainlesshematuriaoradditionallywithfrequenturinationandurinaryurgency,itisofimportancetodetectbladdercanceratearlystagesamonghigh-riskpopulationstoavoidradicalcystoprostatectomyandtoreducebladdercancer-relatedmortality.
Currently,urinecytologyandcystoscopyarethegoldstandardmethodsforcollectinglaboratoryevidencetoaidbladdercancerdiagnosis.Asanon-invasivemethod,cytologicalexaminationispreferablyperformedonvoidedurinesamplesorbladder-washingsamplestodetectexfoliatedcellswithpathologicallyabnormalcharacteristics.However,thismethodsuffersfromlowsensitivityandlargevariations,especiallyforlow-gradetumors.Cystoscopyis,ontheotherhand,aninvasivemethodtoobservetumorlesionsontheinternalwallofcystofpatientswithsuspectedbladdercancer.However,thismethodcausessignificantdiscomfort,andbladdercarcinomainsitumaygounder-detected8.Bladdertumorantigen(BTA)statandBTAtraktests,whichdetecturinebiomarkers,haveshowntoreportwithpoorsensitivityandselectivityforthediagnosisofbladdercancer9.ImmunoCytisfluorescence-basedcytologywiththeaidofacocktailofmonoclonalantibodies.Italsosuffersfromlowsensitivity(68.3鈥?6.5%)andspecificity(62.9鈥?8.5%)10.UroVysionisaFISH-basedassayfordetectionofP16tumorsuppressorgeneinchromosomes3,7,9and17inexfoliatedcellsinurine.Thisassayalsohaslowsensitivity(75.6%)andspecificity(84.8%)11.Clearly,accuratediagnosticmethodsarelackingfordiagnosisofbladdercancerduringearlystagesforscreening.
Recently,studieshaveshownthatEVsorexosomesisolatedfrombiologicalsamplessuchasplasma,urine,salivaandcerebrospinalfluidscanbeusedforcancerdiagnosisandtreatmentmonitoring12,13.However,thestandardmethodforisolationofEVs(i.e.ultracentrifugation)istime-consuming(6鈥?鈥塰),labor-intensive,andinstrument-dependent.Alternativemicrofluidics-basedExoChips14andPolydimethylsiloxane(PDMS)devices15havebeendevelopedforisolationofEVsfromserumorplasma.Forexample,EVsderivedfrompancreaticcancerpatientswerecapturedbyCD63antibody,whichwasimmobilizedonExoChips.ThecapturedEVswerethenstainedwithfluorescence,whichrevealedthatthelevelofexosomesfromthecancergroupwassignificantlyhigherthanhealthyindividuals14.Inanotherstudy,PDMSdevicesisolatedandenrichedEVsfromnon-small-celllungcancerpatientsorovariancancerpatientsusingmagneticbeads,whichwereconjugatedwithapanelofsurfacebiomarkers(i.e.,EpCAM,CA125,伪-IGF-1R,CD9,CD81andCD63)15.SubsequentchemicallysisofEVson-chipenabledanalysisofintravesicularbiomarkersbyELISA,showingthatnon-small-celllungcancerpatientshadasignificantlyelevatedlevelofIGF-1Rthanhealthyindividuals.Thesestudieshaveclearlydemonstratedthefeasibilityofdevelopingmicrofluidicdevicesforisolation,enrichmentandanalysisofEVsfrombiologicalsamplesderivedfromcancerpatients.
Inthismanuscript,wedevelopedanintegrateddouble-filtrationmicrofluidicdeviceforisolation,enrichmentandquantificationofurinaryEVswithasizerangeof30鈥?00鈥塶mfrombladdercancerpatients.Basedontheprincipleofsize-exclusion,twopolycarbonatemembraneswithaporesizesof200or30鈥塶mwereusedtofractionandenrichEVswithinthissizerange.ThecapturedEVswerethenanalyzedusingon-chipELISAaswepreviouslyreported16,17,whichgreatlystreamlinedtheprocessforpoint-of-care(POC)testing.OurresultsshowedthatbladdercancerpatientshadasignificantlyelevatedlevelofurinaryEVswithinthesizerangeof30鈥?00鈥塶mthanhealthycontrolswithasensitivityof81.3%ataspecificityof90%.Thedouble-filtrationdevicedevelopedinthisproof-of-conceptstudycanbebroadlyappliedtoisolateurinaryEVs,orEVswithadifferentsizerangetoscreenforgenitourinarycanceratthePOC.
MaterialsandMethodsExperimentalreagentsandchemicalsWhatman庐Nuclepore鈩?track-etchedmembraneswithaporesizeof30or200鈥塶mwerepurchasedfromGEHealthcareLifeScience(Shanghai,China).Poly(methylmethacrylate)(PMMA)anddouble-sideadhesive(DSA)wereobtainedfrom3鈥塎Company(St.Paul,Minnesota).100-and500-nmfluorescentnanoparticles(FNPs)werepurchasedfromOceanNanotech,LLC(Springdale,CA).Biotinylatedanti-CD63antibody,streptavidin-labeledhorseradishperoxidase(HRP),andanti-CD9antibodywereobtainedfromAbcamInc.(Cambridge,MA).3,3鈥?5,5鈥?Tetramethylbenzidine(TMB),lyophilizedbovineserumalbumin(BSA),andphosphatebufferedsaline(PBS,pH7.0)werepurchasedfromSangonBiotechCo.,Ltd.(Shanghai,China).TheBicinchoninicAcidKitand0.22鈥壩糾syringefilterswerealsoobtainedfromSangonBiotechCo.,Ltd.(Shanghai,China).
DevicefabricationDeviceswereassembledwiththeaidofalasercutteraspreviouslyreported18,19.Thedouble-filtrationdevice,whichhadanouterdimensionof20鈥壝椻€?0鈥壝椻€?鈥塵m,wasassembledusingfourlayersofPMMA,fourlayersofDSAand2filtermembranes(Fig.1A).ThethicknessoffourPMMAlayerswas2,1,1and2鈥塵m,respectively.ThethicknessofDSAwas50鈥壩糾.PMMAandDSAwereexcisedusingaUniversalLasersystem(UniversalLaserSystemInc.,Scottsdale,AZ).OnthefirstPMMAlayer,twocircularopeningswithadiameterof1.8鈥塵mwereexcisedtoallowforsampleinjectionandwastecollection.ThesecondPMMAlayerhadtwoseparatecircularchamberswithadiameterof10鈥塵m.ThethirdPMMAlayerconsistedoftwoseparatecircularchamberswithadiameterof10鈥塵m,whichwereconnectedthroughamicrochannelwithawidthof1.5鈥塵m.TwoWhatmanmembraneswithaporesizeof200鈥塶mand30鈥塶mwereassembledbetweenthesecondandthirdPMMAlayer(Fig.1A).TherewerenoopeningsonthefourthPMMAlayer.AtotaloffourlayersofDSAwereusedtoassemblethedoublefiltrationdevice,formingtwochambers(78.5鈥壩糒)abovethemembranesandtwochambers(78.5鈥壩糒)belowthemembranes.DevicesIandII(Fig.2A),whichcontainedasinglemembranewithaporesizeofeither200鈥塶mor30鈥塶m,werealsopreparedtovalidatethedouble-filtrationdevice.
Figure1:IsolationanddetectionofEVsfromurineusinganintegrateddouble-filtrationmicrofluidicdevice.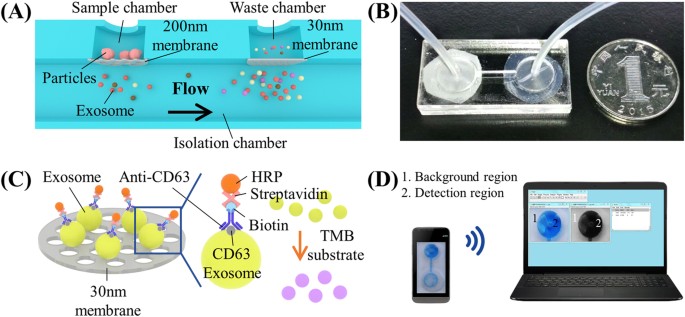
(A)Schematicofadouble-filtrationmicrofluidicdeviceforisolationanddetectionofEVs.Basedonsize-exclusion,particleslargerthan200鈥塶mareexcludedbythemembranewithaporesizeof200鈥塶minthesamplechamber,whereasparticlessmallerthan30鈥塶mpassthroughthedouble-filtrationdevice.EVswithasizebetween30and200鈥塶mareisolatedandenrichedintheisolationchamber.(B)Imageofanassembleddouble-filtrationdevice.(C)SchematicofdirectELISAforEVdetectionon-chip.TheEVsisolatedinthedouble-filtrationdevicearelabeledwithbiotinylatedanti-CD63antibodies,andthenwithstreptavidin-HRP.AdditionofTMBsubstrateenablesbluecolordevelopmentinthedouble-filtrationdevice.(D)TheELISAresultisimagedusingasmartphoneandthentransferredtoalaptopfordataanalysisusingImageJ.
FullsizeimageFigure2:Validationofsize-exclusionfordouble-filtrationdevices.
(A)SchematicofDevicesIandIItomimictheprimaryandsecondaryfiltrationprocessofthedouble-filtrationdevice.Amixtureof500and100鈥塶mFNPswasflowedthroughDeviceI(containeda200鈥塶mpore-sizedmembraneonly).ThefiltratecollectedfromthewastechamberofDeviceIwastheninjectedintoDeviceII(containeda30鈥塶mpore-sizedmembraneonly).Thenumberof500and100鈥塶mFNPscollectedfromthefiltrateswasmeasuredwiththeaidofafluorescencemicroscope.(B)Anscanningelectronmicroscopy(SEM)imageofa200鈥塶mpore-sizedmembrane(scalebar1鈥壩糾).(C)AnSEMimageofa30鈥塶mpore-sizedmembrane(scalebar1鈥壩糾).(D)Atypicalimageof500鈥塶mand100鈥塶mFNPs.(E)RecoveryratesofFNPscollectedatthewastechambersofDevicesIandII.
FullsizeimageDevicecharacterizationToinvestigatetheperformanceofthedouble-filtrationdevice,amixtureof100and500鈥塶mFNPswassequentiallyinjectedintoDevicesIandIIusingamicropump(LongerCo.,Ltd,Baoding,China).500鈥壩糒ofthemixturewasfirstinjectedintoDeviceIcontainingasinglemembranewithaporesizeof200鈥塶m,and500鈥壩糒ofairwasthenflowedthroughthedevice.TheresultantfiltratenamedFiltrateIwascollectedfromthewastechamberusingatubingwithaninnerdiameterof0.5鈥塵m.FiltrateIwassubsequentlyinjectedtoDeviceII,whichcontainedasinglemembranewithaporesizeof30鈥塶m,and500鈥壩糒ofairwasflowedthroughthedevice.TheresultantfiltratenamedFiltrateIIwascollectedfromthewastechamber.Finally,thenumberof100and500鈥塶mFNPswascountedunderafluorescencemicroscope(LeicaDM4000,German)toverifytheprecisionofsize-exclusionofthedouble-filtrationmicrofluidicdevice.
CellcultureHumanbladdercancercelllineT24wasculturedinRPMI1640mediumsupplementedwith10%heat-inactivatedfetalbovineserum(FBS),100鈥塙/mLpenicillin,and100鈥壩糶/mLstreptomycin(Shanghai,China)at37鈥壜癈inahumidatmospherewith5%CO2.Cellmediumwasharvestedaftercellcultureforthreedays,andthenumberofcellswascountedusingahemocytometer(MolecularDevices,SanFrancesco,CA).
UrinesamplesTheuseofdiscardedurinesampleswasapprovedbyanexpeditedInstituteofReviewBoard(IRBNo.2016253)atTheFirstAffiliatedHospital,CollegeofMedicine,ZhejiangUniversity.Discardedurinesamples(30鈥塵L)werecollectedfrom16patientswhowereadmittedtothehospitalwithconfirmativediagnosisofbladdercanceronthisexpeditedIRBfromwhichdetailedpatientinformationsuchasdiseasestageandsexwasexempted.Noexclusioncriteriawerespecified.Asacontrol,8urinesampleswerecollectedfromhealthysubjectsandtested.Alltheurinesampleswereprocessedwithin6鈥塰oursuponcollection.
IsolationofEVsusingultracentrifugation100鈥塵LofT24cellculturemediawasharvestedonDay4.Cells,celldebrisandmicrovesicles(MVs)wereremovedbycentrifugingat20,000鈥?i>gatroomtemperaturefor15鈥塵inutes.Theresultantsupernatantwasfilteredthroughacommercial0.22鈥壩糾filterdevicetoremovepotentiallycontaminatedbacteria.Thefiltratewasthencentrifugedat100,000鈥?i>gat4鈥壜癈for70鈥塵inutesinatypeSW32TirotortoprecipitateEVs.ThecrudeEV-containingpelletsweresuspendedin1鈥塵LofPBSaftersupernatantwascarefullyremoved.TocollectEVsfromhumanurine,100鈥塵Lofurinesampleswasprocessedasdescribedabove.AlltheisolatedEVswerekeptat鈭?0鈥壜癈untilfurtheruse.
IsolationofEVsusingdouble-filtrationmicrofluidicdevicesUrinesamplesandT24culturemediawerecentrifugedat20,000鈥?i>gatroomtemperaturefor15鈥塵inutes.Theresultantsupernatantwasthenfilteredthroughacommercial0.22鈥壩糾filterdevicetoremovepotentiallycontaminatedbacteria.8鈥塵Loftheobtainedfiltratewasinjectedintoadouble-filtrationdeviceataflowrateof40鈥壩糒/minandittookapproximately200鈥塵intocompletethefiltrationprocess.Thedouble-filtrationdevicewasthenwashedwith400鈥壩糒ofPBSbufferataflowrateof40鈥壩糒/minforthreetimes.Thedouble-filtrationmicrofluidicdevicewasthenflowedwith500鈥壩糒ofairtocompletelyremoveresidualliquid.
Dynamiclightscattering(DLS)analysisDynamiclightscatteringanalysisofisolatedEVswasperformedusingaZetasizerNanoS-90Instrument(Malvern,Worcestershire,UK)atambienttemperature.Thedetectionanglewas90掳andthewavelengthofhelium/neonlaserwas633鈥塶m.ThesizeofEVswasdeterminedaccordingtotheStokes鈥揈insteinequation,andthesizedistributionofEVswascharacterized.
ProteinquantificationAbicinchoninicacid(BCA)kit(SangonBiotech,Shanghai,China)wasusedtoestimatetheconcentrationofEV-associatedproteinisolatedfromurineandcellculturemedia.Toestablishaquantificationcurve,5鈥壩糒ofBSAstandardsolutions(0,0.025,0.075,0.125,0.25,0.5,0.75,and1鈥塵g/mL)wasmixedwith5鈥壩糒ofSolutionFina96-wellplateandthenincubatedat37鈥壜癈for30鈥塵inutes.200鈥壩糒ofBCAworkingsolutionwasthenaddedtothe96-wellplate,whichwasincubatedat37鈥壜癈for30鈥塵inutes.Theabsorbancewasmeasuredatawavelengthof562鈥塶musingaspectrophotometer(MolecularDevices,SanFrancesco,CA).5鈥壩糒ofEVs,whichwasisolatedfromurineorculturemediabyultracentrifugationanddouble-filtration,wastestedinparallelwiththeBSAstandards.TheconcentrationofEV-associatedproteinwascalculatedbydeductionoftheamountofproteininthefiltrateafterdouble-filtrationfromthetotalamountofproteininurinebeforedouble-filtration.
QuantificationofEVsusing96-wellplateELISATheprocedureof96-wellplateELISAincluded:i)100鈥壩糒ofEVsuspensions(undiluted,seriallydilutedto1:10,1:100,1:1,000,1:10,000and1:100,000)wasaddedtoa96-wellplateandthenincubatedat4鈥壜癈overnight,ii)200鈥壩糒ofBSA(1%)wasaddedtoeachwelltoexcludeunboundactivesitesat37鈥壜癈for1鈥塰our,iii)300鈥壩糒ofPBS(pH7.0)bufferwasusedtowasheachwellforthreetimes,iv)100鈥壩糒ofbiotinylatedanti-CD63antibody(1:200,5鈥壩糶/mL)wasaddedtoeachwellandthenincubatedat37鈥壜癈for1鈥塰our,v)300鈥壩糒ofPBS(pH7.0)wasusedtowasheachwellforthreetimes,vi)100鈥壩糒ofstreptavidin-labeledHRP(1:2,000)wasaddedtoeachwellandthenincubatedat37鈥壜癈for1鈥塰our,vii)300鈥壩糒ofPBS(pH7.0)wasusedtowasheachwellforthreetimes,viii)100鈥壩糒ofTMBwasaddedtoeachwellandincubatedat37鈥壜癈for10鈥塵inutesindarkness,ix)50鈥壩糒ofstopsolutionwasthenaddedtoeachwelltoterminatefurthercolordevelopment,andx)theopticaldensityofeachwellwasmeasuredatawavelengthof450鈥塶musingaspectrophotometer(MolecularDevices,SanFrancesco,CA).PBSwasusedasnegativecontrolin96-wellplateELISA.Forquantification,10arbitraryunitsweredefinedforthecolorimetricintensityobtainedinthelowestserialdilution(i.e.,1:100,000dilution).
QuantificationofEVsusingmicrochipELISATheprocedureofmicrochipELISAforquantificationofEVsisolatedusingadouble-filtrationdeviceincluded:i)300鈥壩糒ofEVsuspensionsresultedfromultracentrifugation(seriallydilutedto1:10,1:100,1:1,000,and1:10,000)wasinjectedtodouble-filtrationdevices,ii)300鈥壩糒ofbiotinylatedanti-CD63antibodies(1:200)wasinjectedintothedouble-filtrationdevicesataflowrateof40鈥壩糒/minandthenincubatedat25鈥壜癈for1鈥塰our,iii)300鈥壩糒ofPBSwasusedtowashthedevicesforthreetimes,iv)500鈥壩糒ofairwasinjectedtocompletelyremoveliquid,v)300渭Lofstreptavidin-labeledHRP(1:2,000,0.5鈥壩糶/mL)wasinjectedtothedevicesandincubatedat37鈥壜癈for1鈥塰ourinawetbox,vi)thedeviceswerewashedasStepsiiiandiv,vii)300鈥壩糒ofTMBsubstratesolutionwasinjectedintothedevicesandincubatedat37鈥壜癈for10鈥塵inutesindarkness,viii)thedevelopmentofbluecoloronchipwasimagedusingacellphone(R7,OPPO,Dongguan,China),ix)theimagesweretransferredviawirelesscommunicationtoalaptopforImageJprocessing,andx)redchannelvaluesofbluecolorasaresultofELISAwereusedtoconstructastandardcurveaspreviouslyreported16,17.PBS,asnegativecontrol,wastestedinparallelwithEVsamplesinmicrochipELISA.
ScanningelectronmicroscopyandtransmissionelectronmicroscopyThemembranewithaporesizeof200and30鈥塶mindiameterwasobservedunderascanningelectronmicroscope(SEM)(Hitachi,Tokyo,Japan).Briefly,membraneswerecutintoasizeof5鈥壝椻€?鈥塵mandfixedin2.5%glutaraldehydeovernightat4鈥壜癈.ThemembraneswerethenimmersedinPBS(0.1鈥塎,pH7.0)forthreetimes(15鈥塵inutespertime).Themembranesweresubsequentlyfixedinosmicacid(1%)for1鈥塰our.ThemembraneswereimmersedinPBSforthreetimesasthepreviouswashingstep.Themembraneswereprocessedwithgradientconcentrations(30%,50%,70%,80%,90%and95%)ofaceticacid,andthenwashedwith100%ofethanoltwice(20鈥塵inutespertime).Themembraneswerethentreatedwiththemixtureofethanolandaceticacid(V/V鈥?鈥?:1)for30鈥塵inutes,followedbypureaceticacidfor1鈥塰our.ImageswerecapturedunderSEMaftercriticalpointdryingandgoldsputtering.
TheEVs,isolatedfromurinebyultracentrifugation,werevisualizedusingtransmissionelectronmicroscopy(TEM)(Hitachi,Tokyo,Japan)accordingtothefollowingsteps:5鈥壩糒ofEVsuspensionwasfixedin50鈥壩糒ofparaformaldehyde(2%),and2鈥壩糒ofthismixturewasaddedontotheformvar-carboncoatedelectronmicroscopygridsfor3鈥塵inutes.3鈥壩糒ofosmicacidwasthenaddedfornegativestainingfor1鈥塵inute.Aftertheliquidwasdriedbylenspaper,thecoppergridswereplacedunderTEMforimaging.
ClinicaltestingandstatisticalanalysisTwenty-fourdiscardedclinicalurinesampleswereobtainedfromTheFirstAffiliatedHospital,CollegeofMedicine,ZhejiangUniversity(IRBNo.2016253).TheconcentrationofEVswasquantifiedusingmicrochipELISAandthenlog-transformed.Box-WhiskeranalysiswasperformedusingOrigin8.0(OriginLab,Massachusetts,USA).Receiveroperatingcharacteristic(ROC)curvewasplottedforassessmentofsensitivityandspecificity.Atwo-sidedStudent鈥檚t-testwasperformedusingIBMSPSSV22(NewYork,US),inwhichpvaluelessthan0.05wasconsideredstatisticallysignificant.
ResultsWorkingprincipleoftheintegrateddouble-filtrationmicrofluidicdeviceToisolateandenrichEVsfromurinesamples,adouble-filtrationmicrofluidicdevicewasdevelopedbasedonsize-exclusion(Fig.1A,B).Embeddedinthedevicearetwomembraneswithporesizesof200and30鈥塶mindiameter.Accordingtotheworkingprincipleofsize-exclusion,particleslargerthan200鈥塶mareexcludedbythemembranewithaporesizeof200鈥塶minthesamplechamber,whereasparticlessmallerthan30鈥塶mpassthroughthedevicetothewastechamber.EVswithasizebetween30鈥?00areisolatedandenrichedintheisolationchamber.Sincealargervolumeofsamples(e.g.,8鈥塵L)canbecontinuouslyflowedthroughthedouble-filtrationmicrofluidicdevice,EVsareenrichedintheisolationchamberinasmallervolume(i.e.,165鈥壩糒).FollowingisolationandenrichmentofEVsintheisolationchamber,EVsaredetectedusingadirectmicrochipELISA(Fig.1C).TheisolatedEVsarelabeledwithbiotinylatedanti-CD63antibodies,andtheresultantimmuno-complexsubsequentlyinteractswithstreptavidin-HRP.AdditionofTMBsubstratetothedeviceresultsinbluecolordevelopment,whichisimagedusingasmartphone.Theimagesarethenwirelesslytransferredtoalaptopforimageprocessinganddataanalysis(Fig.1D).
Characterizationofthedouble-filtrationdeviceTocharacterizethedouble-filtrationdevice,DevicesIandII,whichmimickedtheprimaryandsecondaryfiltrationstepsofthedouble-filtrationdevice,werealsoassembled,containingasinglemembranewith200or30鈥塶mpores,respectively(Fig.2A).Theporesizeoffiltrationmembraneswasapproximately200鈥塶m(Fig.2B)or30鈥塶m(Fig.2C)indiameterconfirmedunderSEM.Amixtureof100and500鈥塶mFNPswassequentiallyflowedthroughDevicesIandIIataflowrateof40鈥壩糒/min.Atypicalimageofthemixtureof100and500鈥塶mFNPswasshowninFig.2D.Figure2Eshowsthattherecoveryof500鈥塶mFNPsfromthefiltratewas0%forbothDevicesIandII,andthattherecoveryof100鈥塶mFNPsfromthefiltratewas92.0%and0%forDevicesIandII,respectively.Takentogether,thesedatashowedthat500and100鈥塶mFNPswereeffectivelyexcludedbythefiltrationmembranewithporesizesof200and30鈥塶m,respectively,indicatingthatEVswithasizerangingfrom30鈥?00鈥塶mcouldbeisolatedandenrichedbythedouble-filtrationmicrofluidicdevice.
OptimizationofflowratesandcharacterizationofEVsToisolateEVsfromurineviadoublefiltration,increasingflowrates(e.g.,20,30,40and50鈥壩糒/min)wereinvestigated.Sinceitwasobservedthataflowrateof50鈥壩糒/mincausedabulgeonthe200鈥塶mporesizedmembranewhenfilteringurinesamples,flowratessuchas20,30,and40鈥壩糒/minwerefurtherassessedbycheckingtheintactnessofEVsusingDLSbeforeandafterdouble-filtration.Priortodouble-filtrationofurinesamples,DLSanalysisshowedasymmetricunimodaldistributionofurinaryEVsinsizerangingfrom68.1to295鈥塶m.TheaveragesizeofEVsafterdouble-filtrationwas155鈥塶mandthepeakdiameterwas105鈥塶m(Fig.3A).TherewasnosignificantdifferenceinpeaksizeofEVsobtainedattheflowrateof20,30,or40鈥壩糒/min(Fig.3B).Additionally,nosmallervesiclesweredetectedinthefiltrateofurineafterdouble-filtrationattheflowrateof20,30,or40鈥壩糒/min.ThedatasuggestedthatEVsmaintainedtheirintactnessduringdouble-filtrationundertheseflowconditions,andthatmostEVswithinthesizerangeof30鈥?00鈥塶mpassedthroughthe200鈥塶mmembraneandcondensedintheisolationchamber.Afterevaluationandoptimization,theflowrateof40鈥壩糒/minwasusedforisolatingEVsfromurineusingdouble-filtrationmicrofluidicdevices.
Figure3:CharacterizationofEVsisolatedfromurineusingadouble-filtrationdevice.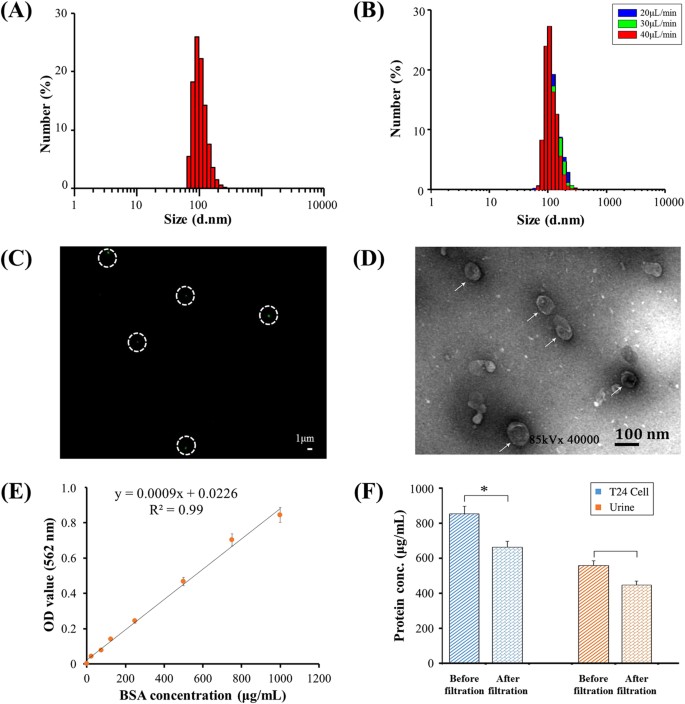
(A)SizedistributionofurinaryEVspriortodoublefiltration.(B)SizedistributionofurinaryEVsafterdoublefiltrationataflowrateof20,30or40鈥壩糒/min.(C)EVswerestainedwithAlexaFluo庐488-labeledanti-CD9antibody,andthenobservedunderafluorescencemicroscope.Scalebaris1鈥壩糾.(D)ThemorphologyandsizeofEVswereobservedunderTEM.Scalebaris100鈥塶m.(E)StandardcurveoftheBCAmethodforproteinquantification.(F)QuantificationofEV-associatedprotein.EVsisolatedfrom8鈥塵LofT24cellculturemediaor8鈥塵Lofurinecollectedfromahealthydonorusingultracentrifugationwerethenflowedthroughdouble-filtrationdevices.TheconcentrationoftotalproteinbeforeandafterdoublefiltrationwasquantifiedusingtheBCAmethod.Atwo-sidedStudent鈥檚t-testwasperformedusingSPSSV22.Theasterisk(*)indicatesstatisticalsignificance(p鈥?lt;鈥?.05).
FullsizeimageEVsisolatedfromurinewerefurthercharacterizedusingfluorescencestainingandTEM.Figure3CshowsatypicalfluorescenceimageofEVslabeledwithantibodyagainstCD9,whichisoneofthecharacteristicproteinbiomarkersofEVs20,21.InFig.3,thepresenceoffluorescence-stainedEVswasobservedasgreendotsinwhitecircles.ThemorphologyandsizeofEVswerefurtherinvestigatedwiththeaidofTEM.EVsindicatedbywhitearrowswereclosetoacircularshapewithadiameterofapproximately100鈥塶m(Fig.3D).Inaddition,theconcentrationofproteinpresentincellmediaorurinebeforeandafterdouble-filtrationwascharacterizedusingtheBCAmethod(Fig.3E).TheconcentrationofproteinpresentinT24culturemediawas853.4鈥壩糶/mL,anditwasreducedto662.7鈥壩糶/mLafterdouble-filtration;theconcentrationofproteinpresentinurinewas556.9鈥壩糶/mL,anditwasreducedto446.8鈥壩糶/mLafterdouble-filtration(Fig.3F),indicatingthattheconcentrationofEV-associatedproteinwas190.7鈥壩糶/mLand110.1鈥壩糶/mLforT24culturemediaandurine,respectively.Takentogether,thedouble-filtrationmicrofluidicdevicedemonstratesthecapabilitytoisolateEVsfromculturemediaandurine.
QuantificationofEVsusingmicroplateandmicrochipELISAToquantifyEVs,bothmicroplateandmicrochipELISAweredeveloped.Thelowestdetectionpointwasdefined10arbitraryunit(AU)ofT24cellculturemedia,andthestandardcurveofmicroplateELISAwasshownasFig.4AwithR2of0.97.Accordingtothestandardcurve,theconcentrationofEVsfromahealthycontrol鈥檚urinesamplewasquantified,yielding34,9818.7鈥堿U/mL.Astandardcurvewasalsoconstructedusingthisurinesample,withadetectionlimitof35.0鈥堿U/mLasshowninFig.4B.TheseresultsindicatethatEVscouldbereliablyquantifiedfromT24cellculturemediaandurineusingadirectmicroplateELISA.ToquantifyEVsdirectlyinthedouble-filtrationdevice,microchipELISAwasalsodevelopedwithalinearityof0.90(R2)overtherangeof100to1,000,000鈥堿U/mL(Fig.4C).BasedonthestandardcurveofmicrochipELISA,theconcentrationofEVsenrichedbyultracentrifugationanddouble-filtrationfrom8鈥塵LofT24culturemediawas190,541.5(AU/mL)and141,298.7(AU/mL),respectively.Thus,theisolationandenrichmentefficiencyofEVsusingthedouble-filtrationdevicewas74.2%comparedtoultracentrifugation(Fig.4D).
Figure4:DevelopmentofmicrochipELISAfordetectionofEVs.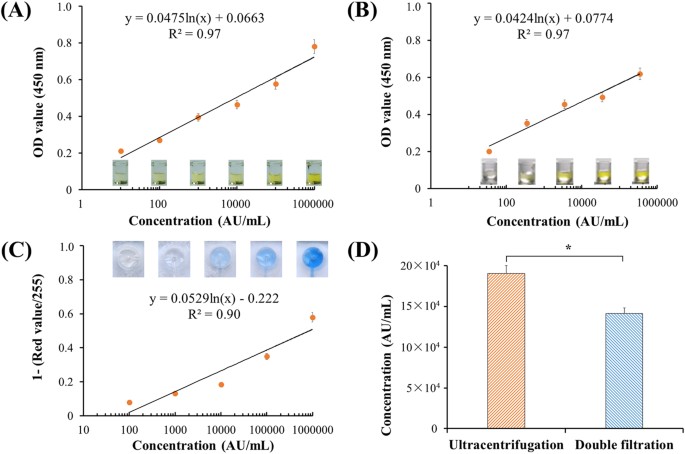
(A)StandardcurveofmicroplateELISAfordetectionofEVsfromcellculture.EVswereisolatedfrom8鈥塵LofT24cellmediausingultracentrifugationandwere10-folddiluted.Thelowestdetectionpointwasdefinedas10鈥堿U/mL.Side-viewimagesof96-wellmicroplateELISAwereshown.(B)DetectionofurinaryEVsusingmicroplateELISA.EVswereisolatedfrom8鈥塵LofurinefromahealthydonorusingultracentrifugationandsubsequentlyquantifiedusingmicroplateELISA.(C)StandardcurveofmicrochipELISAfordetectionofEVs,whichwereisolatedfrom8鈥塵LofT24cellmediausingultracentrifugation.ImagesofmicrochipELISAwerepresentedforeachstandardconcentration.(D)ComparisonofisolationefficiencyofEVsbetweenultracentrifugationanddouble-filtration.TheconcentrationofEVswasquantifiedusingmicrochipELISA.Atwo-sidedStudent鈥檚t-testwasperformedusingSPSSV22.Theasterisk(*)indicatesstatisticalsignificance(p鈥?lt;鈥?.05).
FullsizeimageClinicalvalidationofthedouble-filtrationdeviceTovalidatethedouble-filtrationdevice,urinesamplesfrombladdercancerpatients(n鈥?鈥?6)andnormaldonors(n鈥?鈥?)wereapplied,andtheconcentrationofEVswasmeasuredusingmicrochipELISA(Fig.5A).TheresultsshowedthattheconcentrationofurinaryEVsfrombladdercancerpatientswasinawiderangefrom32,790.0to368,284.0(AU/mL),whereastheconcentrationofurinaryEVsfromhealthycontrolswas16,458.4to41,279.6(AU/mL).TheseresultsindicatethaturinaryEVsweresuccessfullyisolatedusingthedouble-filtrationdevice,anddetectedbysubsequentmicrochipELISA.Moreimportantly,itwasfoundthattheconcentrationofurinaryEVsfrombladdercancerwassignificantlyhigherthanthatfromhealthycontrols(Fig.5A).Inaddition,areceiveroperatingcharacteristic(ROC)curvewasplottedforclinicalvalidation.AsshowninFig.5B,themicrochipELISAhadasensitivityof81.3%atthespecificitysetto90.0%foridentifyingbladdercancerpatientsfromhealthycontrols,andtheAUROCwas0.96.
Figure5:ClinicalvalidationofurinaryEVsfordifferentiatingbladdercancerpatientsfromhealthyindividuals.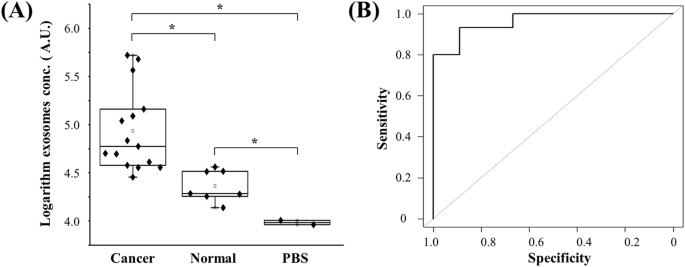
(A)EVswereisolatedandenrichedfromurinesamplescollectedfrombladdercancerpatients(n鈥?鈥?6)andhealthycontrols(n鈥?鈥?),andsubsequentlytestedusingmicrochipELISA.Thelog-transformedEVconcentrationsinbladdercancerpatientsandhealthycontrolswerecomparedinabox-plot.Atwo-sidedStudent鈥檚t-testwasusedtoanalyzethestatisticaldifferencebetweenthetwogroups.Theasterisk(*)indicatesstatisticalsignificance(p鈥?lt;鈥?.05).(B)Receiveroperatingcharacteristic(ROC)curvewasplottedforassessmentofsensitivityandspecificity.Thesensitivity,specificityandtheareaunderROCcurve(AUROC)wereanalyzedusingSPSSV22.TheresultsdemonstratedthatthisintegratedEVdouble-filtrationdevicehadasensitivityof81.3%ataspecificityof90%.
FullsizeimageDiscussionInthisstudy,wedevelopedanintegrateddouble-filtrationmicrofluidicdevice,whichisolates,enrichesandquantifiesurinaryEVson-chiptoassistinscreeningforbladdercanceratthePOC(Fig.1).Thedevelopeddouble-filtrationdevicewasbasedonsize-exclusiontoisolateEVswithsizerangingfrom30to200鈥塶m.Fordevicecharacterization,amixtureof500and100鈥塶mFNPswassequentiallyflowedthroughDevicesIandIIcontaining200and30鈥塶mpore-sizedmembranes,whichmimickedtheprimaryandsecondarymodulesofthedouble-filtrationdevice(Fig.2A).Theresultsclearlyshowedthat500鈥塶mFNPswereexcludedbythe200-nmpore-sizedmembraneandthat100鈥塶mFNPswereretainedbythe30鈥塶mpore-sizedmembrane(Fig.2E),indicatingthatEVscanbeisolatedandenrichedbysettingupsizethresholdsbetween30and200鈥塶m.Indeed,fluorescencestainingsuccessfullydetectedoneofthehallmarkmolecules(i.e.,CD9)ofEVsisolatedfromurinesamplesandT24cellculturemediausingthedouble-filtrationdevicebasedonsize-exclusion(Fig.3C).Furthermore,themorphologyofEVsisolatedfromurineorcellculturemediashowedroundorellipsoidalparticleswithasizerangingfrom40鈥?00鈥塶m(Fig.3D),confirmingthatthedouble-filtrationdevicebasedonsize-exclusioncanbereliablyusedtoisolateandenrichEVsfromurineorcellculturemedia.
Thedouble-filtrationmicrochipapproachrecovered74.2%ofEVscomparedtoultracentrifugation,whichismoderatelylowerthanapreviousstudybasedonasequentialfiltrationstrategyforisolatingEVs(81%)withthesizeof40鈥?00fromcellculture22.Thelowerisolationefficiencyofdoublefiltrationcomparedtoultracentrifugationmayresultfromthefollowingpossiblereasonsortheircombination.The,impuritiessuchaslargerextracellularvehiclesmayattributetotheoverestimationofEVsisolatedbyultracentrifugation,whichisbasedonthegravitydifferenceamongEVsaftermultipleroundsofcentrifugationatvaryingspeeds.ThepresenceofEVswithasizelargerthan200鈥塶mwouldcontributetheoverestimationofEVsisolatedfromultracentrifugationbyELISA,whichinturnwouldlowertheisolationefficiencyofEVsusingdouble-filtration.AlthoughEVs,whicharenanoscaleparticleswithbilipidmembranes,maybesqueezedandbrokenintoundetectableparticlesduringthedouble-filtrationprocess,DLSanalysisofthefiltrateafterdoublefiltrationdidnotdetectparticles.Additionally,nosmallervesiclesweredetectedinthefiltrateofurineafterdouble-filtrationattheflowrateof20,30,or40鈥壩糒/min(Fig.S1).Thus,themoderatelylowerrecoveryefficiencyofEVsfromdoublefiltrationismostlylikelyduetothepresenceofimpuritiesinEVsprecipitatedbyultracentrifugation.
Tamm-Horsfallprotein(THP),asanabundantproteininhumanurine,mayaffecttheisolationanddetectionofEVsinthisdouble-filtrationbasedmicrochipELISA.Inanearlierstudy,urinesamplesweretreatedwithareducingagent,dithiothreitol(DTT),whichwasfollowedbyre-ultracentrifugationtoremoveabundantTHPfromurineforanalyzinglow-abundanceproteins23.However,theuseofDTTtodenatureTHPisalsoofaconcern,sinceitcanalsodenatureotherproteinsincludingactiveproteinbiomarkersontheoutersurfaceofEVmembranes24.Thus,DTTwasexcludedfromurinetreatmentforprofilingproteinbiomarkersfromurinaryEVsbymassspectrophotometry24.Similarly,DTTwasnotusedtoremoveTHPfromurine,sincesurfacebiomarkerCD63-basedELISAwasutilizedforquantificationofurinaryEVs.Inaddition,THPismainlysecretedbytheloopofHenleatthethickascendinglimbinkidney25,26andformsintopolymericfilamentsinurine27.AlthoughpolymericTHPfilamentsmaytrapsomeEVs,thesetrappedEVswouldbemorelikelyderivedfromrenalcorpusclesorsecretedbykidney.SincethisportionofEVswouldlesslikelyaddclinicallyrelevantdiagnosticvaluesforassessingtherisksofbladdercancer,weexcludedtheuseofDTTtoremoveTHPfromurineintheCD63-basedmicrochipELISAforanalyzingurinaryEVsderivedfrombladdercancerpatients.
Inthisstudy,weaimedatdevelopinganintegrateddeviceforisolatingandquantificationofEVsfromurineforinitialscreeningofbladdercanceratthePOC.Couplingon-chipELISAwithisolationandenrichmentofEVsinaninexpensive,disposabledevicesignificantlystreamlinetheanalysisofurinaryEVsatthePOC.Moreimportantly,theconcentrationofEVsfrombladdercancerpatientswassignificantlyelevatedcomparedtohealthycontrols,andmicrochipELISAdifferentiatedbladdercancerpatientsfromhealthycontrolswithasensitivityof81.3%ataspecificityof90%(Fig.5).Althoughbladdercanceriscurrentlydiagnosedusingcytologyincombinationwithcystoscopyandhistology,theyarelimitedbyinvasiveexaminationandpoorsensitivity.Non-invasiveapproachestoassessingurinarybiomarkersofbladdercancer(e.g.,NMP22,BTAandurinarysurviving)generallylacksensitivityforlow-gradebladdercancer,andtheseurinarybiomarkersmaybefalselyelevatedduetonon-malignantconditionsandhematuria28.Thepresenteddouble-filtrationdeviceforanalyzingtheconcentrationofurinaryEVsrepresentsanattractiveapproachtoaidinitialscreeningofbladdercanceratthePOC.
FromaperspectiveofPOCtesting,thedevelopedEVquantificationmodalityisadvantageousovertraditionalmethodsforisolatinganddetectingEVsfromurinesamples,consideringinsufficientinfrastructureandinexperienceoperatorsinPOCsettings29,30.Thedouble-filtrationdeviceeliminatedtheneedforanultracentrifugetoisolateEVsfromurine,whichiscostlyandtime-consuming.Theprincipleofcellphone-basedon-chipELISA,whichwepreviouslydeveloped16,17,wasusedinthisstudytoaccommodateconvenientdetection/quantificationofurinaryEVswithoutreferencetoaspectrophotometer.ComparedtootherexistingEVanalyticalmethodssuchasExoChipsandimmunoisolation-baseddevices,ourintegratedEVanalyticaldeviceismorecost-effective.SinceExoChipreliesontheuseofantibodiesimmobilizedonthesurfaceofmicrochannelstocaptureEVsfromserum,itiscostlybecauseoftheuseoflargeamountofantibodies,anditmaysufferfromextensiveassessmentofflowratetoachieveoptimalsensitivityandspecificity.Incontrast,ourdouble-filtrationdeviceisbasedonsize-exclusion,whicheliminatestheuseofcaptureantibodytoisolateEVsandobviatesthetediousoptimizationofflowrate.Immunoisolation-baseddevices,albeitofshorterassaytime(e.g.,1.5鈥塰),requiressophisticatedanalyticaltoolssuchasuprightepi-fluorescencemicroscopetoanalyzetheplasma-derivedexosomesfromnon-small-celllungcancerpatients,whichisnotpracticaloruser-friendlyforPOCtesting.Thus,ourintegrated,inexpensive,anddisposablemicrofluidicdeviceisadvantageousovercurrentexosomeandEVanalyticalmethodsforPOCtesting.
ConclusionInconclusion,wedevelopedadouble-filtrationdeviceforisolationandenrichmentofEVsfromurineandsubsequentdetectionviamicrochipELISAwiththeaidofacellphone.Thisintegratedapproachdemonstratedtheproof-of-conceptofusingtheconcentrationofEVstodifferentiatebladdercancerfromhealthycontrolsinanon-invasivemanner.Inthisapproach,EVsareisolatedandenrichedbasedonsize-exclusionbymembranes,eliminatingtheneedforimmuno-captureon-chipandsignificantlysimplifyingmicrochipELISAatthePOC.Furthermore,thisplatformandcellphonesystemhavepotentialtobeintegratedwithadvancesensortechnologies,includingsurfaceplasmonresonancetools,nanomechanicalplatforms,andelectricalsensingsystems31,32,33.Nevertheless,futurestudieswillbenefitfromclinicalvalidationinalargerpopulationandapplicationsinothergenitourinarycancers.
AdditionalInformationHowtocitethisarticle:Liang,L.-G.etal.Anintegrateddouble-filtrationmicrofluidicdeviceforisolation,enrichmentandquantificationofurinaryextracellularvesiclesfordetectionofbladdercancer.Sci.Rep.7,46224;doi:10.1038/srep46224(2017).
Publisher"snote:SpringerNatureremainsneutralwithregardtojurisdictionalclaimsinpublishedmapsandinstitutionalaffiliations.
References1
Goebell,P.J.Knowles,M.A.Bladdercancerorbladdercancers?Geneticallydistinctmalignantconditionsoftheurothelium.UrolOncol28,409鈥?28,doi:10.1016/j.urolonc.2010.04.003(2010).
ArticlePubMedGoogleScholar2Jemal,A.etal.GlobalCancerStatistics.Ca-aCancerJournalforClinicians61,69鈥?0,doi:10.3322/caac.20107(2011).
ArticlePubMedGoogleScholar3Siegel,R.L.,Miller,K.D.Jemal,A.Cancerstatistics,2015.CACancerJClin65,5鈥?9,doi:10.3322/caac.21254(2015).
ArticleGoogleScholar4Babjuk,M.etal.EAUguidelinesonnon-muscle-invasiveurothelialcarcinomaofthebladder:update2013.EurUrol64,639鈥?53,doi:10.1016/j.eururo.2013.06.003(2013).
ArticleGoogleScholar5Chen,W.etal.CancerstatisticsinChina,2015.CACancerJClin66,115鈥?32,doi:10.3322/caac.21338(2016).
ArticlePubMedPubMedCentralGoogleScholar6Kaufman,D.S.,Shipley,W.U.Feldman,A.S.Bladdercancer.Lancet374,239鈥?49,doi:10.1016/S0140-6736(09)60491-8(2009).
CASArticlePubMedGoogleScholar7Bethesda.SEERCancerStatFacts:BladderCancer.CanceroftheUrinaryBladder.http://seer.cancer.gov/statfacts/html/urinb.html(30/04/2016)(2016).
8Steinberg,G.BlueLightCystoscopyShouldbeUsedRoutinelyforBladderCancerDetection.JournalofUrology195,1652鈥?653,doi:10.1016/j.juro.2016.03.073(2016).
ArticlePubMedGoogleScholar9Miyake,M.etal.UrinaryBTA:indicatorofbladdercancerorofhematuria.WorldJournalofUrology30,869鈥?73,doi:10.1007/s00345-012-0935-9(2012).
CASArticlePubMedPubMedCentralGoogleScholar10He,H.,Han,C.,Hao,L.Zang,G.ImmunoCyttestcomparedtocytologyinthediagnosisofbladdercancer:Ameta-analysis.OncologyLetters12,83鈥?8,doi:10.3892/ol.2016.4556(2016).
CASArticlePubMedPubMedCentralGoogleScholar11Dimashkieh,H.etal.EvaluationofUroVysionandCytologyforBladderCancerDetection.CancerCytopathology121,591鈥?97,doi:10.1002/cncy.21327(2013).
ArticlePubMedPubMedCentralGoogleScholar12Skog,J.etal.GlioblastomamicrovesiclestransportRNAandproteinsthatpromotetumourgrowthandprovidediagnosticbiomarkers.NatCellBiol10,1470鈥?476,doi:10.1038/ncb1800(2008).
CASArticlePubMedPubMedCentralGoogleScholar13Zhang,Y.Wang,X.F.Anicheroleforcancerexosomesinmetastasis.Nat.CellBiol.17,709鈥?11(2015).
CASArticleGoogleScholar14Kanwar,S.S.,Dunlay,C.J.,Simeone,D.M.Nagrath,S.Microfluidicdevice(ExoChip)foron-chipisolation,quantificationandcharacterizationofcirculatingexosomes.LabonaChip14,1891鈥?900,doi:10.1039/c4lc00136b(2014).
CASArticlePubMedPubMedCentralGoogleScholar15He,M.,Crow,J.,Roth,M.,Zeng,Y.Godwin,A.K.Integratedimmunoisolationandproteinanalysisofcirculatingexosomesusingmicrofluidictechnology.LabonaChip14,3773鈥?780,doi:10.1039/c4lc00662c(2014).
CASArticlePubMedPubMedCentralGoogleScholar16Wang,S.etal.Micro-a-fluidicsELISAforrapidCD4cellcountatthepoint-of-care.SciRep4,3796,doi:10.1038/srep03796(2014).
CASArticlePubMedPubMedCentralGoogleScholar17Wang,S.etal.IntegrationofcellphoneimagingwithmicrochipELISAtodetectovariancancerHE4biomarkerinurineatthepoint-of-care.LabChip11,3411鈥?418,doi:10.1039/c1lc20479c(2011).
CASArticlePubMedPubMedCentralGoogleScholar18Wang,S.etal.Simplefiltermicrochipforrapidseparationofplasmaandvirusesfromwholeblood.Internationaljournalofnanomedicine7,5019鈥?028,doi:10.2147/IJN.S32579(2012).
ArticlePubMedPubMedCentralGoogleScholar19Wang,S.etal.Efficienton-chipisolationofHIVsubtypes.LabChip12,1508鈥?515,doi:10.1039/c2lc20706k(2012).
CASADSArticlePubMedPubMedCentralGoogleScholar20Kalamvoki,M.,Du,T.Roizman,B.Cellsinfectedwithherpessimplexvirus1exporttouninfectedcellsexosomescontainingSTING,viralmRNAs,andmicroRNAs.ProcNatlAcadSciUSA111,E4991鈥?996,doi:10.1073/pnas.1419338111(2014).
CASADSArticlePubMedGoogleScholar21Yoshioka,Y.etal.Ultra-sensitiveliquidbiopsyofcirculatingextracellularvesiclesusingExoScreen.NatCommun5,3591,doi:10.1038/ncomms4591(2014).
CASADSArticlePubMedPubMedCentralGoogleScholar22Heinemann,M.L.etal.Benchtopisolationandcharacterizationoffunctionalexosomesbysequentialfiltration.JournalofChromatographyA1371,125鈥?35,doi:10.1016/j.chroma.2014.10.026(2014).
CASArticlePubMedGoogleScholar23Pisitkun,T.,Shen,R.F.Knepper,M.A.Identificationandproteomicprofilingofexosomesinhumanurine.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica101,13368鈥?3373,doi:10.1073/pnas.0403453101(2004).
CASADSArticlePubMedPubMedCentralGoogleScholar24Hildonen,S.,Skarpen,E.,Halvorsen,T.G.Reubsaet,L.Isolationandmassspectrometryanalysisofurinaryextraexosomalproteins.ScientificReports6,doi:10.1038/srep36331(2016).
25Garimella,P.S.etal.Urinaryuromodulin,kidneyfunction,andcardiovasculardiseaseinelderlyadults.KidneyInternational88,1126鈥?134,doi:10.1038/ki.2015.192(2015).
CASArticlePubMedPubMedCentralGoogleScholar26Rampoldi,L.,Scolari,F.,Amoroso,A.,Ghiggeri,G.Devuyst,O.Therediscoveryofuromodulin(Tamm-Horsfallprotein):fromtubulointerstitialnephropathytochronickidneydisease.KidneyInternational80,338鈥?47,doi:10.1038/ki.2011.134(2011).
CASArticlePubMedGoogleScholar27Fernandez-Llama,P.etal.Tamm-Horsfallproteinandurinaryexosomeisolation.KidneyInt77,736鈥?42,doi:10.1038/ki.2009.550(2010).
ArticlePubMedPubMedCentralGoogleScholar28Schmitz-Drager,B.J.etal.Molecularmarkersforbladdercancerscreening,earlydiagnosis,andsurveillance:theWHO/ICUDconsensus.UrolInt94,1鈥?4,doi:10.1159/000369357(2015).
CASArticlePubMedGoogleScholar29Wang,S.etal.Advancesinaddressingtechnicalchallengesofpoint-of-carediagnosticsinresource-limitedsettings.Expertreviewofmoleculardiagnostics16,449鈥?59,doi:10.1586/14737159.2016.1142877(2016).
CASArticlePubMedPubMedCentralGoogleScholar30Wang,S.Q.,Inci,F.,DeLibero,G.,Singhal,A.Demirci,U.Point-of-careassaysfortuberculosis:Roleofnanotechnology/microfluidics.Biotechnologyadvances31,438鈥?49,doi:10.1016/j.biotechadv.2013.01.006(2013).
ArticlePubMedPubMedCentralGoogleScholar31Inci,F.etal.Multitarget,quantitativenanoplasmonicelectricalfield-enhancedresonatingdevice(NE2RD)fordiagnostics.ProcNatlAcadSciUSA112,E4354鈥?363,doi:10.1073/pnas.1510824112(2015).
CASArticlePubMedGoogleScholar32Inci,F.etal.Nanoplasmonicquantitativedetectionofintactvirusesfromunprocessedwholeblood.ACSNano7,4733鈥?745,doi:10.1021/nn3036232(2013).
CASArticlePubMedPubMedCentralGoogleScholar33Tokel,O.etal.Portablemicrofluidicintegratedplasmonicplatformforpathogendetection.SciRep5,9152,doi:10.1038/srep09152(2015).
CASArticlePubMedPubMedCentralGoogleScholarDownloadreferences
AcknowledgementsDr.WangacknowledgesthesupportedfromtheNationalKeyResearchandDevelopmentProgram(2016YFC1101302)fromMinistryofScienceandTechnologyofthePeople鈥檚RepublicofChina.Dr.DemirciwouldliketoacknowledgeR01AI093282,R01GM108584,R01DE02497101,R01AI081534,R21Al113117,R21Al110277,U54EB015408,DODLC15065011976867StanfordCanaryCenterseedgrant,andStanfordCenterforCancerNanotechnologyExcellenceandTranslationalDiagnostics(CCNE-TD)GrantU54CA199075.
AuthorinformationAffiliationsStateKeyLaboratoryforDiagnosisandTreatmentofInfectiousDiseases,FirstAffiliatedHospital,CollegeofMedicine,ZhejiangUniversity,Hangzhou,310003,ZhejiangProvince,ChinaLi-GuoLiang,聽Meng-QiKong,聽Ye-FengSheng,聽TaoYu,聽Lan-JuanLi聽聽ShuQiWangCollaborativeInnovationCenterforDiagnosisandTreatmentofInfectiousDiseases,Hangzhou,310003,ZhejiangProvince,ChinaLi-GuoLiang,聽Meng-QiKong,聽Ye-FengSheng,聽TaoYu,聽Lan-JuanLi聽聽ShuQiWangInstituteforTranslationalMedicine,ZhejiangUniversity,Hangzhou,310029,ZhejiangProvince,ChinaLi-GuoLiang,聽Meng-QiKong,聽Ye-FengSheng,聽TaoYu聽聽ShuQiWangDepartmentofRadiology,Bio-AcousticMEMSinMedicine(BAMM)Laboratory,CanaryCenteratStanfordforCancerEarlyDetection,StanfordUniversity,SchoolofMedicine,PaloAlto,94304,CA,USASherryZhou,聽FatihInci,聽UtkanDemirci聽聽ShuQiWangDepartmentofUrology,FirstAffiliatedHospital,CollegeofMedicine,ZhejiangUniversity,Hangzhou,310003,ZhejiangProvince,ChinaPingWangHarvardCatalyst-LaboratoryforInnovativeTranslationalTechnologies,HarvardMedicalSchool,Boston,02115,MA,USAWinstonPatrickKuoCloudHealthGenomics,Ltd,Shanghai,201499,ChinaWinstonPatrickKuoDepartmentofElectricalEngineering(Bycourtesy),StanfordUniversity,Stanford,94305,CA,USAUtkanDemirciAuthorsLi-GuoLiangViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarMeng-QiKongViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarSherryZhouViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarYe-FengShengViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarPingWangViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarTaoYuViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarFatihInciViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarWinstonPatrickKuoViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarLan-JuanLiViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarUtkanDemirciViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarShuQiWangViewauthorpublicationsYoucanalsosearchforthisauthorinPubMedGoogleScholarContributionsLi-GuoLiang,UtkanDemirciandShuQiWangdesignedthestudy;Li-GuoLiangandMeng-QiKongperformedtheexperiments,Li-GuoLiang,Meng-QiKongandShuQiWanganalyzedthedata;Li-GuoLiang,UtkanDemirciandShuQiWangwrotethemanuscript;SherryZhou,Ye-FengSheng,PingWang,TaoYu,WinstonPatrickKuo,FatihInci,andLan-JuanLialsocontributedtotheexperimentaldesign,andresultdiscussion.
CorrespondingauthorsCorrespondencetoUtkanDemirciorShuQiWang.
EthicsdeclarationsCompetinginterestsDr.UDemirciisafounderof,andhasanequityinterestin:(i)DxNowInc.,acompanythatisdevelopingmicrofluidicandimagingtechnologiesforpoint-of-carediagnosticsolutions,and(ii)KoekBiotech,acompanythatisdevelopingmicrofluidicIVFtechnologiesforclinicalsolutions.U.D.鈥檚interestswereviewedandmanagedinaccordancewiththeconflictofinterestpolicies.
RightsandpermissionsThisworkislicensedunderaCreativeCommonsAttribution4.0InternationalLicense.Theimagesorotherthirdpartymaterialinthisarticleareincludedinthearticle鈥檚CreativeCommonslicense,unlessindicatedotherwiseinthecreditline;ifthematerialisnotincludedundertheCreativeCommonslicense,userswillneedtoobtainpermissionfromthelicenseholdertoreproducethematerial.Toviewacopyofthislicense,visithttp://creativecommons.org/licenses/by/4.0/
ReprintsandPermissions
AboutthisarticleLiang,L.,Kong,M.,Zhou,S.etal.Anintegrateddouble-filtrationmicrofluidicdeviceforisolation,enrichmentandquantificationofurinaryextracellularvesiclesfordetectionofbladdercancer.SciRep7,46224(2017).https://doi.org/10.1038/srep46224
Downloadcitation
Received:28October2016
Accepted:13March2017
Published:24April2017
DOI:https://doi.org/10.1038/srep46224
JessicaSierra,Jos茅Marrugo-Ram铆rez,RomenRodriguez-Trujillo,M貌nicaMirJosepSamitierSensors(2020)
S.Busatto,A.Zendrini,A.Radeghieri,L.Paolini,M.Romano,M.PrestaP.BergeseBiomaterialsScience(2020)
Comments
BysubmittingacommentyouagreetoabidebyourTermsandCommunityGuidelines.Ifyoufindsomethingabusiveorthatdoesnotcomplywithourtermsorguidelinespleaseflagitasinappropriate.
SignupfortheNatureBriefingnewsletter鈥?whatmattersinscience,freetoyourinboxdaily.
================ 蚂蚁淘在线 ================
免责声明:本文仅代表作者个人观点,与本网无关。其创作性以及文中陈述文字和内容未经本站证实,对本文以及其中全部或者部分内容、文字的真实性、完整性、及时性本站不做任何保证或承诺,请读者仅作参考,并请自行核实相关内容
版权声明:未经蚂蚁淘在线授权不得转载、摘编或利用其他方式使用上述作品。已经经本网授权使用作品的,应该授权范围内使用,并注明“来源:蚂蚁淘在线”。违反上述声明者,本网将追究其相关法律责任。





