

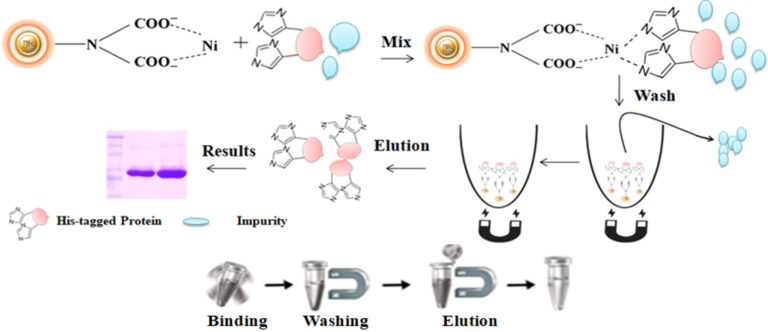
Workflow
The purification with magnetic microparticles is straightforward. Mix the microparticles with the cell lysate and incubate them with continuous rotation for a sufficient time. During mixing, the beads remain suspended in the sample solution, allowing the target molecules to interact with the immobilized ligand. After incubation, the beads are collected and separated from the sample using a magnet rack. Then the ultrapure His-tagged recombinant proteins are eluted by imidazole.
Features and Advantages
●
Quick, Easy, and one-step high-throughput procedure; eliminates columns or filters or a laborious repeat of pipetting or centrifugation.
●
Stable covalent bond with minimal ligand leakage
●
High binding capacity, very low nonspecific binding
●
Scalable – Easily adjusts for sample size and automation
●
Reproducible results
Applications
●
Investigating protein structure and function
●
Preparing recombinant proteins for X-ray crystallography
●
Ideal for study of protein interactions with protein or DNA
●
Immunization to raise antibodies against a protein of interest
●
Effective screening protein expression even with crude cell lysates
●
Microscale purification of his-tagged proteins.
Background information
A polyhistidine tag, also called 6xHis-tag, His-tagged, and His-tag, is a versatile tool for purifying the highly purified recombinant protein from various expression systems, including bacterial, yeast, plant cell, and mammalian cells systems. The tag comprises six or more consecutive histidine amino acid residues positioned at either N or C terminus of a recombinant protein. Due to its small size, His-tag has several distinctive features, including less immunogenicity, hydrophilic nature, and versatility under native and denaturing conditions. Additionally, it is unnecessary to cleave the tag from the recombinant protein since it rarely perturbs the structure and function of its fusion protein. The purification principle of the His-tag depends on immobilized metal ion affinity chromatography (IMAC).
Immobilized metal ion affinity chromatography (IMAC) is a rapid affinity purification chromatography where the his-tagged protein are separated based on their affinity for Ni2+ or Co2+that have been immobilized by a chelator to a solid matrix such as beaded agarose or column. At pH 7-8, his tagged protein will bind to Ni2+ or Co2+. The binding reaction with the tagged protein is affected by various independent variable factors such as pH, temperature, salt type, salt concentration, immobilized metal and ligand density, and protein size. The bound protein is eluted by a decreasing pH gradient, increasing imidazole concentration, or adding an EDTA chelator in a buffer. This technique is an ideal tool for capturing and purification of his-tagged recombinant protein in a quick, inexpensive, and straightforward manner.Although IMAC is a very effective protein purification technique, they are mainly based on traditional affinity chromatography matrices such as agarose resin or column. These solid matrices make the purification process tedious, time-consuming, unable to handle very tiny samples, and challenging to adapt to the automation system. We developed an extremely efficient magnetic IMAC separation system to overcome these limitations.
Learn More
Instruction Manual
MSDS
Recombinant Protein & Purification Related Products →General Reference
1.
Jansen, J-C. (Editor). (2011). Protein Purification: Principles, High-Resolution Methods, and Applications. 3rd edition. Volume 151 of Methods of Biochemical Analysis. John Wiley & Sons, Hoboken, NJ
2.
Bornhorst, J.A. and Falke, J.J. (2000). Purification of Proteins Using Polyhistidine Affinity Tags. Methods Enzymol. 326: 245-254.
3.
Spriestersbach A, Kubicek J, Schäfer F, Block H, Maertens B. Purification of His-Tagged Proteins. Methods Enzymol. 2015;559:1-15. doi: 10.1016/bs.mie.2014.11.003. Epub 2015 May 4. PMID: 26096499.
4.
Zhang C, Fredericks D, Longford D, Campi E, Sawford T, Hearn MT. Changed loading conditions and lysate composition improve the purity of tagged recombinant proteins with tacn-based IMAC adsorbents. Biotechnol J. 2015 Mar;10(3):480-9. doi: 10.1002/biot.201400463. Epub 2014 Oct 31. PMID: 25303209.
5.
Pina AS, et al. (2014) Affinity tags in protein purification and peptide enrichment: An overview. Methods in molecular biology (Clifton, N.J.) 1129: 147-168.
6.
Young CL, et al. (2012) Recombinant protein expression and purification: A comprehensive review of affinity tags and microbial applications. Biotechnol J 7(5): 620-634.
Bioclone的BcMag™NHS激活的磁珠是均匀的,基于二氧化硅的超顺磁珠,表面涂有高密度NHS(N-羟基琥珀酰亚胺)官能团。珠子用于特异性地缀合含伯胺的配体(图1),因此在偶联之前不需要保护配体中的其他活性基团。耦合快(室温pH 6.5–9 15–30分钟,4°C 4小时)。BcMag™NHS激活的磁珠最适合与大蛋白结合。建议使用BcMagTM长臂(17 atome)NHS激活的磁珠来缀合小肽,因为长臂(21原子)亲水性接头可以减少空间位阻。



