
品牌分类
品牌咨询
联系方式
公司地址
苏州工业园区生物纳米园A4#216
联系电话
4000-520-616 / 18915418616
传真号码
0512-67156496
电子邮箱
info@ebiomall.com
公司网址
https://www.ebiomall.com
蚂蚁淘在线 / 品牌中心 / Phoenix Phamaceuticals / Phoenix Phamaceuticals/INSL5, short A- & B-Chains (Human) - MagBead (Magnetic Bead Linked Antibody)/1 ml/MB-035-70

商品描述
A Circulated Hormone that Potentiates Insulin/GLP-1 Secretion and Plays a Role in Glucose Homeostasis and Obesity
- Abstracts
- Schematics
- More Information
  |  |

Expression and localization of relaxin family peptide receptor 4 in human spermatozoa and impact of insulin-like peptide 5 on sperm functions.
Insulin-like peptide 5 (INSL5) is a member of the insulin superfamily peptide that interacts with the relaxin family peptide receptor 4 (RXFP4). Numerous recent studies have focused on the functional effects of INSL5 on fat and glucose metabolism. Although there is no evidence that the human sperm may be a candidate target of INSL5, it has been detected in mice testis and sperm. Therefore, the present study sought to analyze the localization and expression of RXFP4 on human sperm and determine the efficiency of INSL5 in human sperm. Normal semen samples were incubated in different doses and exposure time periods of INSL5. We analyzed sperm motility by computer-assisted sperm analysis (CASA) and ROS levels by flow cytometry using the MitoSOX™ Red probe. Localization and expression of RXFP4 were assayed by immunofluorescence and RT-PCR, respectively. The results confirmed the presence of RXFP4 in human spermatozoa, which localized in the neck and midpiece of sperm. Nested PCR showed the expression of RXFP4 in human sperm. INSL5 could attenuate generation of mitochondrial ROS at the 1, 10, 30, and 100nmol/L doses. This result was particularly noted in the 30nmol/L treated samples after 4h incubation. Total motility of sperm was significantly preserved in the 100nmol/L after 2h and in 30nmol/L after 4h incubation period. This study, for the first time, clarified the expression and localization of RXFP4 on human sperm and revealed the role of INSL5 in sperm motility and mitochondrial ROS generation in a dose-dependent manner.Yeganeh IS, Taromchi AH, Fathabadi FF, Nejatbakhsh R, Novin MG, Shokri S. Reprod Biol. 2017;17(4):327-332.
Liquid chromatography/mass spectrometry based detection and semi-quantitative analysis of INSL5 in human and murine tissues.
RATIONALE: Insulin-like peptide 5 (INSL5) is a hormone produced by enteroendocrine L-cells in the colon that has recently been implicated in the control of metabolic homeostasis. However, research into its physiology has been hindered by the reported unreliability of commercially available immunoassays and additional detection assays would benefit this emerging field. METHODS: Peptides from purified murine L-cells and homogenates from both human and mouse colonic tissues were extracted by precipitating larger proteins with acetonitrile. Untargeted liquid chromatography/tandem mass spectrometry (LC/MS/MS) analyses, followed by database searching, were used to detect and identify various INSL5 gene derived peptides and characterise their precise sequence. A similar approach was developed to quantify INSL5 levels in primary intestinal culture supernatants after purification and concentration by solid-phase extraction. RESULTS: Mass spectral analysis of purified enteroendocrine cells and tissue homogenates identified the exact sequence of A and B chains of INSL5 endogenously expressed in L-cells. Differences in the endogenously processed peptide and the Swissprot database entry were observed for murine INSL5, whereas the human sequence matched previous predictions from heterologous expression experiments. INSL5was detected in the supernatant of human and mouse primary colonic cultures and concentrations increased after treatment with a known L-cell stimulus. CONCLUSIONS: The first LC/MS/MS-based method capable of the detection and semi-quantitative analysis of endogenous INSL5 using MS-based techniques has been demonstrated. The methodology will enable the identification of stimulants for INSL5 secretion from murine and human primary colonic epithelial cultures.This publication used INSL5 peptides (#035-40 and 035-80) from Phoenix Pharmaceuticals for LC-MS/MS standard.
Kay RG, Galvin S, Larraufie P, Reimann F, Gribble FM. Rapid Commun Mass Spectrom. 2017;31(23):1963-1973.
Development of a selective agonist for relaxin family peptide receptor 3.
Relaxin family peptides perform a variety of biological functions by activating four G protein-coupled receptors, namely RXFP1-4. Among these receptors, RXFP3 lacks a specific natural or synthetic agonist at present. A previously designed chimeric R3/I5 peptide, consisting of the B-chain of relaxin-3 and the A-chain of INSL5, displays equal activity towards the homologous RXFP3 and RXFP4. To increase its selectivity towards RXFP3, in the present study we conducted extensive mutagenesis around the B-chain C-terminal region of R3/I5. Decreasing or increasing the peptide length around the B23-B25 position dramatically lowered the activation potency of R3/I5 towards both RXFP3 and RXFP4. Substitution of B23Gly with Ala or Ser converted R3/I5 in an efficient agonist to a strong antagonist for RXFP3, but the mutants retained considerable activation potency towards RXFP4. Substitution of B24Gly increased the specificity of R3/I5 towards RXFP3 over the homologous RXFP4. The best mutant, [G(B24)S]R3/I5, displayed 20-fold higher activation potency towards RXFP3 than towards RXFP4, meanwhile retained full activation potency at RXFP3. Thus, [G(B24)S]R3/I5 is the best RXFP3-selective agonist known to date. It is a valuable tool for investigating the physiological functions of RXFP3, and also a suitable template for developing RXFP3-specific agonists in future.Wei D, Hu MJ, Shao XX, et al. Sci Rep. 2017;7(1):3230.
Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production.
OBJECTIVE: Insulin-like peptide 5 (INSL5) is a recently identified gut hormone that is produced predominantly by L-cells in the colon, but its function is unclear. We have previously shown that colonic expression of the gene for the L-cell hormone GLP-1 is high in mice that lack a microbiota and thus have energy-deprived colonocytes. Our aim was to investigate if energy deficiency also affected colonic Insl5 expression and to identify a potential role of INSL5. METHODS: We analyzed colonic Insl5 expression in germ-free (GF), conventionally raised (CONV-R), conventionalized (CONV-D) and antibiotic-treated mice, and also assessed the effect of dietary changes on colonic Insl5 expression. In addition, we characterized the metabolic phenotype of Insl5-/- mice. RESULTS: We showed that colonic Insl5 expression was higher in GF and antibiotic-treated mice than in CONV-R mice, whereas Insl5expression in the brain was higher in CONV-R versus GF mice. We also observed that colonic Insl5 expression was suppressed by increasing the energy supply in GF mice by colonization or high-fat feeding. We did not observe any differences in food intake, gut transit or oral glucose tolerance between Insl5-/- and wild-type mice. However, we showed impaired intraperitoneal glucose tolerance in Insl5-/- mice. We also observed improved insulin tolerance and reduced hepatic glucose production in Insl5-/- mice. CONCLUSIONS: We have shown that colonic Insl5 expression is regulated by the gut microbiota and energy availability. We propose that INSL5 is a hormone that could play a role in promoting hepatic glucose production during periods of energy deprivation.This publication used an INSL5 antibody from Phoenix Pharmaceuticals for IHC staining.
Lee YS, De vadder F, Tremaroli V, Wichmann A, Mithieux G, Bäckhed F. Mol Metab. 2016;5(4):263-70.
Identification of important residues of insulin-like peptide 5 and its receptor RXFP4 for ligand-receptor interactions.
Insulin-like peptide 5 (INSL5) is an insulin/relaxin superfamily peptide involved in the regulation of glucose homeostasis by activating its receptor RXFP4, which can also be activated by relaxin-3 in vitro. To determine the interaction mechanism of INSL5 with its receptor RXFP4, we studied their electrostatic interactions using a charge-exchange mutagenesis approach. First, we identified three negatively charged extracellular residues (Glu100, Asp104 and Glu182) in human RXFP4 that were important for receptor activation by wild-type INSL5. Second, we demonstrated that two positively charged B-chain Arg residues (B13Arg and B23Arg) in human INSL5 were involved in receptor binding and activation. Third, we proposed probable electrostatic interactions between INSL5 and RXFP4: the B-chain central B13Arg of INSL5 interacts with both Asp104 and Glu182 of RXFP4, meanwhile the B-chain C-terminal B23Arg of INSL5 interacts with both Glu100 and Asp104 of RXFP4. The present electrostatic interactions between INSL5 and RXFP4 were similar to our previously identified interactions between relaxin-3 and RXFP4, but had subtle differences that might be caused by the different B-chain C-terminal conformations of relaxin-3 and INSL5 because a dipeptide exchange at the B-chain C-terminus significantly decreased the activity of INSL5 and relaxin-3 to receptor RXFP4.Wang XY, Guo YQ, Shao XX, Liu YL, Xu ZG, Guo ZY. Identification of important residues of insulin-like peptide 5 and its receptor RXFP4 for ligand-receptor interactions. Arch Biochem Biophys. 2014;558:127-32.
Insulin-like peptide 5 is an orexigenic gastrointestinal hormone.
The gut endocrine system is emerging as a central player in the control of appetite and glucose homeostasis, and as a rich source of peptides with therapeutic potential in the field of diabetes and obesity. In this study we have explored the physiology of insulin-like peptide 5 (Insl5), which we identified as a product of colonic enteroendocrine L-cells, better known for their secretion of glucagon-like peptide-1 and peptideYY. i.p. Insl5increased food intake in wild-type mice but not mice lacking the cognate receptor Rxfp4. Plasma Insl5 levels were elevated by fasting or prolonged calorie restriction, and declined with feeding. We conclude that Insl5 is an orexigenic hormone released from colonic L-cells, which promotes appetite during conditions of energy deprivation.Grosse J, Heffron H, Burling K, et al. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc Natl Acad Sci USA. 2014;111(30):11133-8.
The insulinotropic effect of insulin-like peptide 5 in vitro and in vivo.
Insulin-like peptide 5 (INSL5), a member of the insulin/relaxin superfamily, can activate the G-protein coupled receptor RXFP4, but its precise biological functions are largely unknown. Recent studies suggest that the INSL5/RXFP4 is involved in the control of food intake and glucose homeostasis. We report here that RXFP4 is present in the mouse insulinoma cell line MIN6 and INSL5 augments glucose-stimulated insulin secretion (GSIS) both in vitro and in vivo. RXFP4 is also expressed in the mouse intestinal L cell line GLUTag and INSL5 is capable of potentiating glucose-dependent glucagon-like peptide-1 (GLP-1) secretion in GLUtag cells. We propose that the insulinotropic effect of INSL5 is probably mediated through stimulation of insulin/GLP-1 secretion and INSL5 may be a potential therapeutic target for type 2 diabetes.Luo X, Li T, Zhu Y, et al. The insulinotrophic effect of insulin-like peptide 5 in vitro and in vivo. Biochem J. 2015;466(3):467-73.
Design and recombinant expression of insulin-like peptide 5 precursors and the preparation of mature human INSL5.
Insulin-like peptide 5 (INSL5) is a recently identified insulin superfamily member. Although it binds to and activates the G-protein coupled receptor, RXFP4, its precise biological function remains unknown. To help determine its function, significant quantities of INSL5 are required. In the present work, three single-chain INSL5 precursors were designed, two of which were successfully expressed in E. coli cells. The expressed precursors were solubilized from inclusion bodies, purified almost to homogeneity by immobilized metal-ion affinity chromatography, and then refolded in vitro. One precursor could be converted to two-chain human INSL5 bearing an extended N-terminus of the A-chain (designated long-INSL5) by sequential Lys-C endoproteinase and carboxypeptidase B treatment. The 6 residue A-chain N-terminal extension of long-INSL5 was subsequently removed by Aeromonas aminopeptidase to yield native INSL5 that was designated short-INSL5. Circular dichroism spectroscopic analysis and peptide mapping showed that the recombinant INSL5s adopted an insulin-like conformation and possessed the expected characteristic insulin-like disulfide linkages. Activity assay showed that both long- and short-INSL5 had full RXFP4 receptor activity compared with chemically synthesized human INSL5. This suggested that extension of the N-terminus of the A-chain of long-INSL5 did not adversely impact upon the binding to or activation of the RXFP4 receptor. However, the single-chain INSL5 precursor was inactive which indicated that a free C-terminus of the B-chain is critical for the activity of INSL5. Our present work thus provides an efficient approach for preparation of INSL5 and its analogs through recombinant expression in E. coli cells.Luo X, Bathgate RA, Zhang WJ, et al. Design and recombinant expression of insulin-like peptide 5 precursors and the preparation of mature human INSL5. Amino Acids. 2010;39(5):1343-52.
INSL5 may be a unique marker of colorectal endocrine cells and neuroendocrine tumors.
Insulin-like peptide 5 (INSL5) is a member of the insulin superfamily, and is a potent agonist for RXFP4. We have shown that INSL5 is expressed in enteroendocrine cells (EECs) along the colorectum with a gradient increase toward the rectum. RXFP4 is ubiquitously expressed along the digestive tract. INSL5-positive EECs have little immunoreactivity to chromogranin A (CgA) and might be a unique marker of colorectal EECs. CgA-positive EECs were distributed normally along the colorectum in INSL5 null mice, suggesting that INSL5 is not required for the development of CgA-positive EECs. Exogenous INSL5 did not affect the proliferation of human colon cancer cell lines, and chemically-induced colitis in INSL5 null mice did not show any significant changes in inflammation or mucosal healing compared to wild-type mice. In contrast, all of the rectal neuroendocrine tumors examined co-expressed INSL5 and RXFP4. INSL5 may be a unique marker of colorectal EECs, and INSL5-RXFP4 signaling might play a role in an autocrine/paracrine fashion in the colorectal epithelium and rectal neuroendocrine tumors.Mashima H, Ohno H, Yamada Y, Sakai T, Ohnishi H. INSL5 may be a unique marker of colorectal endocrine cells and neuroendocrine tumors. Biochem Biophys Res Commun. 2013;432(4):586-92.
INSL5-Deficient Mice Display an Alteration in Glucose Homeostasis and an Impaired Fertility.
Insulin-like factor 5 (INSL5), a member of the insulin superfamily, is expressed in the colorectum and hypothalamus. To facilitate studies into the role of INSL5, we generated Insl5(-/-) mice by gene targeting. Insl5(-/-) mice were born in the expected Mendelian ratio, reached normal body weight, but displayed impaired male and female fertility that are due to marked reduction in sperm motility and irregular length of the estrous cycle. Furthermore, Insl5(-/-) mice showed impairment in glucose homeostasis with characteristic elevation of serum glucose levels at an advanced age. Glucose and insulin tolerance tests revealed that the increased blood glucose in Insl5(-/-) mice was due to glucose intolerance resulting from reduced insulin secretion. Morphometric and immunohistological analyses revealed that the Insl5(-/-) mice had markedly reduced average islets area and ß-cell numbers. Furthermore, immunohistochemistry showed the expression of INSL5 in enteroendocrine cells in the colorectal epithelium and the presence of its putative receptor relaxin family peptide receptor 4 in pancreatic islet cells. These results suggest the potential role of INSL5 signaling in the regulation of insulin secretion and ß-cell homeostasis.Burnicka-turek O, Mohamed BA, Shirneshan K, et al. INSL5-deficient mice display an alteration in glucose homeostasis and an impaired fertility. Endocrinology. 2012;153(10):4655-65.
Design and recombinant expression of insulin-like peptide 5 precursors and the preparation of mature human INSL5.
Insulin-like peptide 5 (INSL5) is a recently identified insulin superfamily member. Although it binds to and activates the G-protein coupled receptor, RXFP4, its precise biological function remains unknown. To help determine its function, significant quantities of INSL5 are required. In the present work, three single-chain INSL5 precursors were designed, two of which were successfully expressed in E. coli cells. The expressed precursors were solubilized from inclusion bodies, purified almost to homogeneity by immobilized metal-ion affinity chromatography, and then refolded in vitro. One precursor could be converted to two-chain human INSL5 bearing an extended N-terminus of the A-chain (designated long-INSL5) by sequential Lys-C endoproteinase and carboxypeptidase B treatment. The 6 residue A-chain N-terminal extension of long-INSL5 was subsequently removed by Aeromonas aminopeptidase to yield native INSL5 that was designated short-INSL5. Circular dichroism spectroscopic analysis and peptide mapping showed that the recombinant INSL5s adopted an insulin-like conformation and possessed the expected characteristic insulin-like disulfide linkages. Activity assay showed that both long- and short-INSL5 had full RXFP4 receptor activity compared with chemically synthesized human INSL5. This suggested that extension of the N-terminus of the A-chain of long-INSL5 did not adversely impact upon the binding to or activation of the RXFP4 receptor. However, the single-chain INSL5 precursor was inactive which indicated that a free C-terminus of the B-chain is critical for the activity ofINSL5. Our present work thus provides an efficient approach for preparation of INSL5 and its analogs through recombinant expression in E. coli cells.Luo X, Bathgate RA, Zhang WJ, et al. Design and recombinant expression of insulin-like peptide 5 precursors and the preparation of mature human INSL5. Amino Acids. 2010;39(5):1343-52.
Membrane receptors: structure and function of the relaxin family peptide receptors.
The receptors for members of the relaxin peptide family have only recently been discovered and are G-protein-coupled receptors (GPCRs). Relaxin and insulin-like peptide 3 (INSL3) interact with the leucine-rich-repeat-containing GPCRs (LGRs) LGR7 and LGR8, respectively. These receptors show closest similarity to the glycoprotein hormone receptors and contain large ectodomains with 10 leucine-rich repeats (LRRs) but are unique members of the LGR family (class C) as they have an LDL class A (LDLa) module at their N-terminus. In contrast, relaxin-3 and INSL5 interact with another class of type I GPCRs which lack a large ectodomain, the peptide receptors GPCR135 and GPCR142, respectively. These receptors are now classified as relaxin family peptide (RXFP) receptors, RXFP1 (LGR7), RXFP2 (LGR8), RXFP3 (GPCR135) and RXFP4 (GPCR142). This review outlines the identification of the peptides and receptors, their expression profiles and physiological roles and the functional interactions of the peptides with their unique receptors.Kong RC, Shilling PJ, Lobb DK, Gooley PR, Bathgate RA. Membrane receptors: structure and function of the relaxin family peptide receptors. Mol Cell Endocrinol. 2010;320(1-2):1-15.
Synthesis, conformation, and activity of human insulin-like peptide 5 (INSL5).
Insulin-like peptide 5 (INSL5) was first identified through searches of the expressed sequence tags (EST) databases. Primary sequence analysis showed it to be a prepropeptide that was predicted to be processed in vivo to yield a two-chain sequence (A and B) that contained the insulin-like disulfide cross-links. The high affinity interaction between INSL5 and the receptor RXFP4 (GPCR142) coupled with their apparent coevolution and partially overlapping tissue expression patterns strongly suggest that INSL5 is an endogenous ligand for RXFP4. Given that the primary function of the INSL5-RXFP4 pair remains unknown, an effective means of producing sufficient quantities of this peptide and its analogues is needed to systematically investigate its structural and biological properties. A combination of solid-phase peptide synthesis methods together with regioselective disulfide bond formation were used to obtain INSL5. Both chains were unusually resistant to standard synthesis protocols and required highly optimized conditions for their acquisition. In particular, the use of a strong tertiary amidine, DBU, as N(alpha)-deprotection base was required for the successful assembly of the B chain; this highlights the need to consider incomplete deprotection rather than acylation as a cause of failed synthesis. Following sequential disulfide bond formation and chain combination, the resulting synthetic INSL5, which was obtained in good overall yield, was shown to possess a similar secondary structure to human relaxin-3 (H3 relaxin). The peptide was able to inhibit cAMP activity in SK-N-MC cells that expressed the human RXFP4 receptor with a similar activity to H3 relaxin. In contrast, it had no activity on the human RXFP3 receptor. Synthetic INSL5 demonstrates equivalent activity to the recombinant-derived peptide, and will be an important tool for the determination of its biological function.Akhter hossain M, Bathgate RA, Kong CK, et al. Synthesis, conformation, and activity of human insulin-like peptide 5 (INSL5). Chembiochem. 2008;9(11):1816-22.
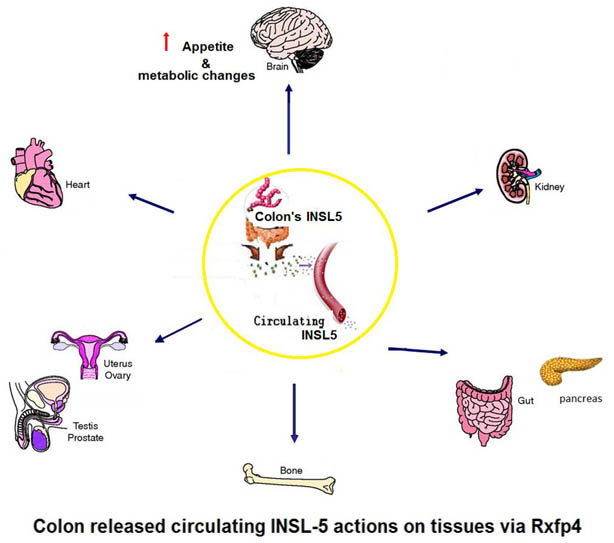
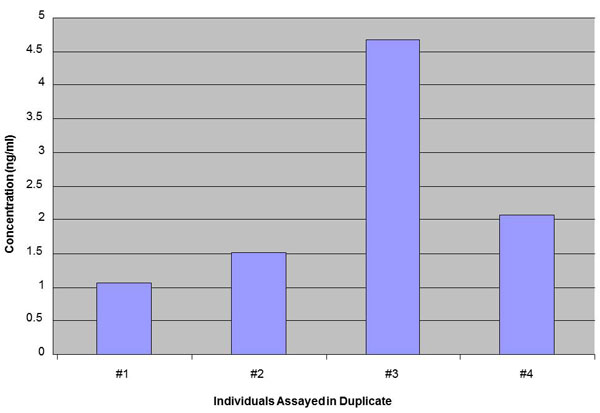
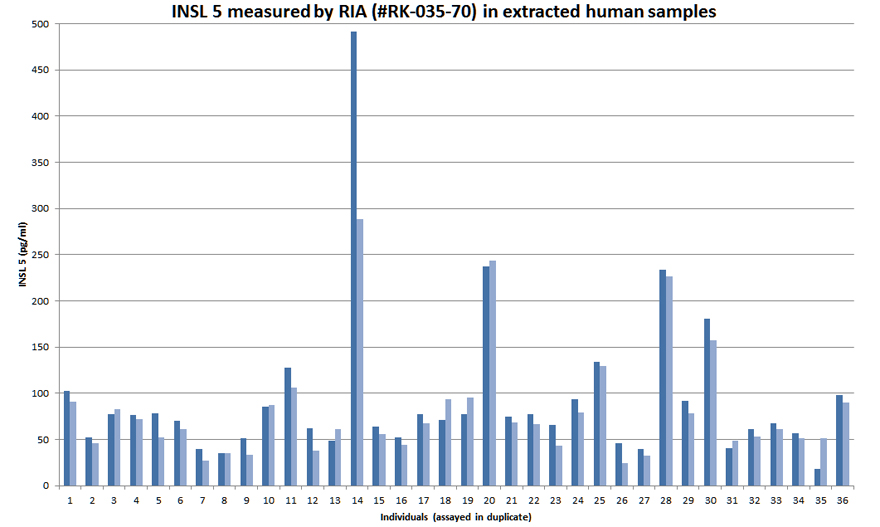
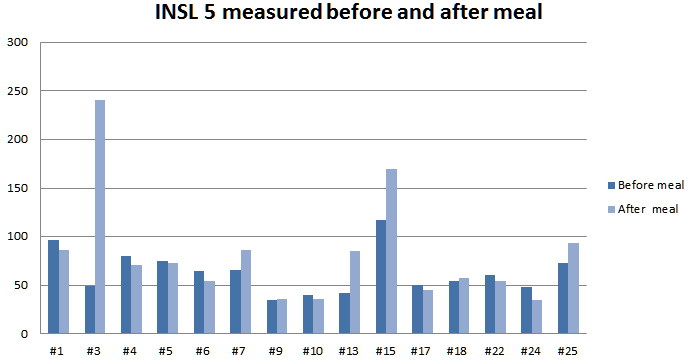
Human Insulin-like 5 (INSL 5) level in Plasma measured by EK-035-70
No References




